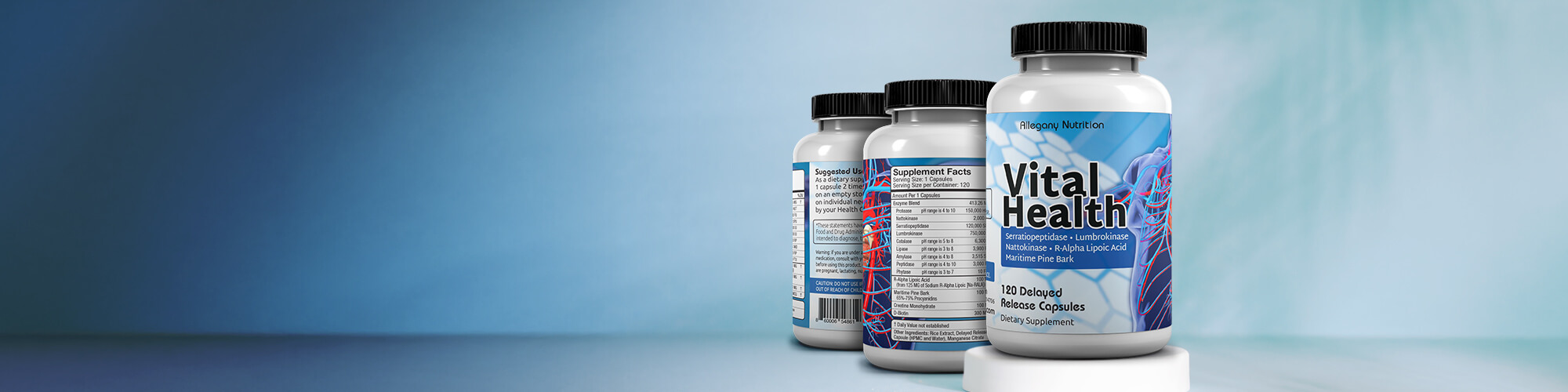
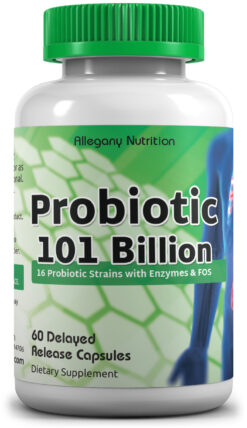
Featured Products
HEALTH CARE PRACTITIONERS
Our product line is sold exclusively to Health Practitioners and Health Food Stores. Examples of Health Practitioners are: Doctors, Chiropractors, ND’s, Massage Therapists, MH, and more. Health students who are currently taking classes or courses are also welcome to purchase our products.
Fast Shipping
We commit 100% to fast customer service and satisfaction. We ship all products within 1 business day!
Money Back Guarantee
We offer a 30 day money back guarantee on every product we sell. If you are not satisfied with any of our products, you may return the unused portion within 30 days of purchase for a refund minus shipping charges and a $10 processing fee.