pH Range Chart
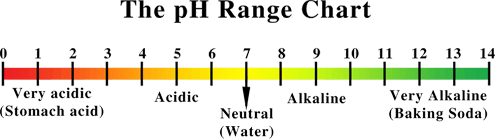
pH stands for “potential hydrogen”. It is by definition the degree of concentration of hydrogen ions in a substance or solution. It is measured on a scale of 0 – 14. The higher numbers mean that a substance is more alkaline and there is a greater potential for absorbing more hydrogen ions. The lower the numbers mean that a substance is more acid (acidic) with less potential for absorbing hydrogen ions. The body’s pH is important because it controls the speed of biochemical reactions. This in effect controls the enzyme activity and the electricity that moves through the body. The higher the pH number (7-14) the more alkaline the substance – the more resistant there is and the slower the electricity will travel. Conversely, the lower the pH number (0-7) the more acidic the substance – the less resistance there is and the faster the electricity will travel.
The Enzyme Comparison Chart
Microbial or fungal enzymes are the only enzymes that digest all of the major food groups in the widest possible pH range while remaining free of any animal content (see below for details).
Where do enzymes come from? Enzymes come from one of three sources: plants, animals, or microbes. Plant enzymes (bromelain and papain) work in a wide pH range but only digest some proteins. Animal enzymes (pepsin, pancreatin) work in a very limited pH range and only digest some of the food groups. The microbial enzymes are the only ones that work in a wide pH range and will digest all of the food groups.

